Electronic Nose
The basis for our approach involves an effort at Caltech that has recently led to arrays of simple, readily fabricated, chemically sensitive conducting polymer films. As illustrated in Figure 1 below, an array of sensors that individually respond to vapors can produce a distinguishable response pattern for each separate type of analyte or mixture (Figure 1). Pattern recognition algorithms and or neural network hardware are used on the output signals arising from the electronic nose to classify, identify, and where necessary quantify, the vapor or odors of concern. This response is much like the way the mammalian olfactory sense produces diagnostic patterns and then transmits them to the brain for processing and analysis.
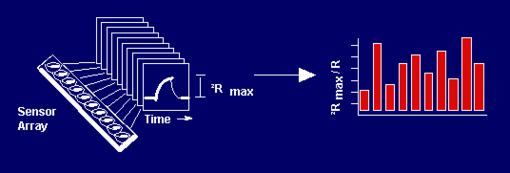
Fig. 1. An array of broadly-cross reactive sensors in which each individual sensor responds to a variety of odors, but the pattern of differential responses across the array produces a unique pattern for each odorant.
This approach does not require development of highly specific recognition chemistries, one for each of the many possible analytes of interest. Instead this approach requires a broadly responsive array of sensors that is trainable to the target signature of interest and then can recognize this signature and deliver it to the sensing electronics in a robust fashion for subsequent processing by pattern recognition algorithms. The Caltech electronic nose functions at atmospheric pressure, functions in a variety of ambients, exhibits near-real time detection, and has already been demonstrated to track vapors in air.
The underlying principle of the Caltech electronic nose is extraordinarily simple. When a polymer film is exposed to a gaseous vapor, some of the vapor partitions into the film and causes the film to swell (Figure 2a). In the electronic nose, this swelling is probed electrically because the sensor films each consist of a composite that contains regions of a conductor that have been dispersed into the swellable organic insulator. The vapor-induced film swelling produces an increase in the electrical resistance of the film because the swelling decreases the number of connected pathways of the conducting component of the composite material. The detector films can be formed from conducting polymer composites, in which the electronically conductive phase is a conducting organic polymer and the insulating phase is an organic polymer, or from polymer-conductor composites in which the conductive phase is an inorganic conductor such as carbon black, Au, Ag, etc and the insulating phase is a swellable organic material. The electrical resistance of the device is then read using simple, low power electronics.
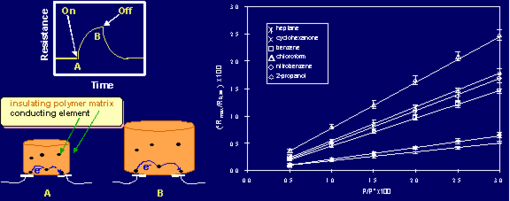
Fig. 2. (a) Swelling occurs as an odorant partitions into the polymer. (b) A linear response of an individual sensor signal as a function of concentration is observed for a variety of analytes.
Any individual sensor film responds to a variety of vapors, because numerous chemicals will partition into the polymer and cause it to swell to varying degrees. However, an array of sensors, containing different polymers, yields a distinct fingerprint for each odor because the swelling properties over the entire array are different for different vapors. The pattern of resistance changes on the array is diagnostic of the vapor, while the amplitude of the patterns indicates the concentration of the vapor. An example of the different patterns produced by various different vapors on the electronic nose is provided in Figure 3.
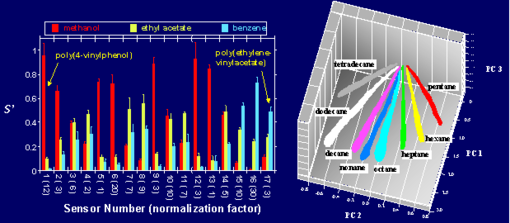
Fig. 3. Response patterns for three different solvents on a 17 element sensor array. (b) Data in principal component space from a 20-detector array exposed to n-tetradecane, n-dodecane, n-decane, n-nonane, n-octane, and n-heptane each at P/Pf = 0.005 to 0.03 in air (with Pf being the vapor pressure of the analyte at 300 K), showing that the pattern type identifies the vapor and the magnitude of the pattern signals is linearly proportional to the analyte concentration.
Work at Caltech has also shown that the array response is a linear function of analyte concentration (Figures 2b, 3b). Since the device functions analogously to the mammalian olfactory system, in that both systems are based on change detection in the surrounding gas composition, the ambient odors in the electronic nose are incorporated into the baseline resistance signals that are recorded before exposure to the vapor of interest. The differential response, relative to this baseline, is the diagnostic odor fingerprint of concern. More specifically, the pattern obtained upon exposure to an analyte of interest is essentially independent of the composition of the background ambient, so that exposure to a given concentration of heptane in air, for example, produces the same response pattern as an exposure to that same concentration of heptane on top of a background of benzene, 2-propanol, water vapor, or a variety of other analytes (Figure 4a). In addition, work at Caltech has shown that the linearity and additivity features of these sensors also apply to binary mixtures of analytes, in that the array response pattern produced by exposure to a binary mixture of analytes is given by the pattern produced by the individual analytes weighted by the relative amounts of each analyte in the vapor mixture (Figure 4b).
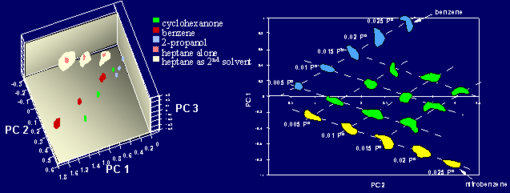
Fig. 4. (a) Data in principal component space from a 12-detector array exposed to different concentrations of various solvents and then to a test solvent, heptane, on top of a background of these other solvents. The pattern observed for heptane is essentially independent of the composition of the background gas. (b) Data in principal component space showing exposures to various concentrations of benzene, nitrobenzene, and benzene/nitrobenzene binary mixtures, illustrating that the patterns of binary mixtures are linear combinations of the patterns obtained from the individual analytes.
In addition, the temperature sensitivity of the baseline resistance is small compared to the differential resistance signal induced by exposure to vapors, so temperature control is not critical to the satisfactory functioning of the system under ambient conditions except under the most stressing of detection conditions. These properties of the Caltech nose, which are not available to date in most other array-based vapor detection systems, allow it to operate in room air and at a variety of humidities. Consequently, the Caltech nose already has been demonstrated to detect odors in an ordinary room background and to direct robotics to turn toward the source of the odor with no assistance from auxiliary devices such as a carrier gas, vacuum pumps, or sampling instrumentation.
Because the swelling of the polymer begins immediately after exposure to the vapor, the resistance signals can be read in real-time or near-real-time. Currently, with films on the order of 1 micron in thickness, the swelling (and therefore resistance) response times to equilibrium film swelling values range from < 0.1 sec to > 100 sec, depending on the vapor and the polymer through which the vapor must diffuse. Characteristic patterns are produced even at times shorter than equilibrium, so the time-dependent swelling properties also provide diagnostic pattern information on the vapor of interest. At present, the pattern is read out after a fixed exposure time, and it is not necessary that equilibrium be reached for all sensors in order to obtain a desired, characteristic fingerprint of the odorant. More rapid responses to equilibrium could, if required, be simply obtained through reduction in the film thickness. Since the diffusion time is proportional to the square of the film thickness (if Fickian diffusion is obeyed), decreasing the film thickness to the range of 0.1 micron should provide real-time responses of most vapors on the electronic nose array. This sensing method is much more rapid, disposable, and inherently inexpensive than can be achieved at present using gas chromatographic methods, than using biosensors that require transfer of particles or vapors into the liquid phase, or than most other approaches to chemical detection of a vapor's presence. These factors highlight the distinct advantages of the electronic nose technology.
At small swellings, the film returns fully to its initial unswollen state after the vapor source is removed, and the film resistance on each array element returns back to its original value. Such reversibility has been demonstrated in the Caltech nose for tens of thousands of vapor exposures, under a variety of ambient conditions in room air at various relative humidities and temperatures, for a diverse set of odors and polymers. Many sensor films have demonstrated stability for months, although this is not even expected to be an operational requirement because the sensor heads could be made so inexpensively that they could be disposed of after a few days or even after a single use if so required.
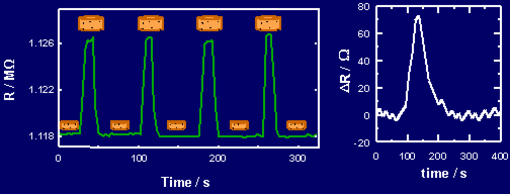
Fig 5. (left) Repeated exposures of a typical sensor to benzene vapor. Fig. 6. (right) Signal of an unoptimized poly(ethylehe-co-vinyl acetate)-carbon black composite to 883 ppb nitrotoluene in air.
The sensitivity of the conducting polymer composite based electronic nose compares highly favorably to other array-based vapor detection systems. For example, Figure 6 shows the response of a single sensor element to 880 ppb of nitrotoluene. This response was limited by the digitizing resolution of the measurement instrumentation, but was still significantly above the noise floor for such chemiresistors. In addition, it did not take advantage of coherent signal detection algorithms over a large number of array elements, which should produce significant increases in sensitivity. Nor did it take advantage of optimized measurement electronics that operate at the optimum frequency to maximize the signal/noise ratio of the sensor element. The sensitivity can also be increased by tailoring the components of the composite, both the insulator and filler, to consist of species that will naturally show an increased affinity towards classes of compounds that are characteristic of analyte families of interest. For example, as described below, the basis for our proposed studies of amine-based biomarkers is the tailoring of sensors to be highly responsive to biogenic amines. Such tailoring has produced a million-fold increase in sensitivity to these specific classes of materials such that we can now detect 10 ppt (10 parts per trillion) or less of biogenic amines in a few seconds merely by static headspace sampling of the gas sample. Another advantage of the polymer-based systems is that their sensitivity is not dictated by the absolute concentration of analyte determining, for example, a count rate in a detector. Instead, the gas-polymer partition coefficient determines the sensitivity of an individual array element to a particular odorant. Because molecules with a low vapor pressure have a thermodynamic tendency to avoid the gas phase, in general such analytes exhibit higher partition coefficients into a given set of polymers than do more volatile compounds, which have an increased propensity to remain in the gas phase. Thus, the polymer-based electronic nose technology affords an inherent sensitivity advantage towards very low vapor pressure compounds in the presence of high vapor pressure materials like very low molecular weight organics, water, methanol, etc.. We intend to exploit this advantageous property of the Caltech electronic nose in certain application areas to be developed in this work.
A key feature of the technology involves the approach of fabricating the chemically sensitive resistors using generic, stable conductive fillers in conjunction with common organic insulating polymers. This allows ready incorporation of a wide range of chemical diversity into the polymer films, and also allows facile control over the electrical properties of the sensor elements through control over the conductor/insulator ratio in an individual chemiresistor. The commercial, off-the-shelf, organic polymers provide the basic sensor components that respond differently to different vapors, based on the differences in polarity, molecular size, and other properties of the vapor. This diversity in gas/polymer binding interactions produces the chemical diversity amongst array elements in the electronic nose sensor head. The ability to use the electrical conductance as a signal makes this mode of sensing very attractive because it is low power and is readily integrated into conventional signal conditioning electronics. Although commercial electronic nose devices currently exist (in their infancy) based on other conducting polymer films, the previous approaches only use sensor films consisting of pure conducting organic polymer phases such as polypyrrole, polyacetylene, or polythiophene and their derivatives. Such films are limited in number chemically, have limited environmental stability, are highly sensitive to variations in humidity, cannot be tuned in their electrical resistance properties through compositional changes in order to mate uniformly with a fixed impedance range of an amplifier and signal conditioning circuit, and have swelling properties that can only be modified slightly chemically. These drawbacks, which greatly reduce not only the diversity of the library and therefore the ultimate breadth in responsivity of the sensor array to complex target signatures, but also reduce the potential of such systems in actual applications outside of the laboratory, are avoided in the Caltech system, which already has distinguished methanol from ethanol from isopropanol, benzene from toluene, acetone from ethyl acetate, R- from S- enantiomers (through incorporation of polymers having chiral functional groups along their backbone), light from heavy water, 51%/49% mixtures of two analytes from 50%/50% mixtures of the same analytes, beer from wine from hard liquor, dead fish from live fish, rose oil from garlic, etc., as well quantifying the composition of binary vapor mixtures, with no active control over humidity or temperature of the ambient or of the sensors, and with demonstrated sensitivities in the sub-ppm range on a single (unoptimized) sensor element (Figure 6). In the near future the system should demonstrate much improved sensitivities on sensor arrays as the coherence of the target analyte signature on the array response is incorporated into data processing algorithms.
At present, the Caltech electronic nose consists of approximately 20-50 different polymeric sensors that are deposited on substrates that are approximately 1 mm-1 cm in size. The electronics are now integrated with the sensor elements, and we have a hand-held unit that can control the sensor temperatures, acquire the data from as many as 32 sensor channels (expandable to 132 easily), and record the resulting resistance vs time data to a laptop PC. There are important performance advantages to be realized, however, in miniaturizing this technology and in integrating the sensor materials, signal conditioning, signal measurement, and signal processing electronics into a monolithic, VLSI-compatible, device framework in either a one chip or two chip solution. These advantages include the increased signal/noise from use of a larger number of sensors, the ability to veto poorly performing sensors based on the response of the rest of the array, and the ability to distinguish background clutter better from the target signature. This will minimize the false alarm rates through use of a highly diverse set of sensors that can probe even very subtle differences between two different odorants. Additional advantages are decrease in measurement time and increased sensitivity from having the sensor elements and electronics integrated and on chip. Moreover there are substantial manufacturing cost advantages of achieving a VLSI-compatible vapor detection system. We are working on these aspects of the technology implementation at the present time in conjunction with NASA and with engineering groups at Caltech who specialize in VLSI and integrated chip design.
Key questions that form topics of interest to the Lewis group:
- Can one map electronic nose smell space onto human odor quality space, i.e. in essence assign a number to a human value judgment of smell?
- Can one utilize electronic nose data to obtain chemical class information on analytes?
- What controls the time dependence of the signals of the carbon black composite detectors?
- How does one effectively calibrate such systems or compensate for drift in response signals?
- How does one obtain generically improved response properties from polymer-based sorption devices?
- Can one construct an electronic system that mimics functionally the complex odorant classification behavior that is characteristic of human olfaction?
- How does the performance of systems with a few different detectors compare to that of a combinatorially-generated array that has hundreds or thousands of different detectors?