Non-conventional Solar Absorbers
Low cost, earth-abundant light absorbers are of great interest for use in terrestrial photovoltaics and in artificial photosynthesis. Non-conventional solar absorbers, e.g., zinc phosphide (Zn3P2), cuprous oxide (Cu2O) and pyrite (FeS2), are promising materials for solar energy conversion and have received renewed interests after early work in 1980s. The Lewis group is interested in studying the interface chemistry and fundamental energy-conversion properties of these non-conventional solar absorbers.
Zinc Phosphide
Zinc phosphide is one of the most promising materials for low-cost solar cells. It has a direct bandgap near 1.5 eV, both of its constituent elements are cheap and earth abundant and electron diffusion length in p-type Zn3P2 is relatively long (>10 um).
We have successfully demonstrated synthesis of quasi-single crystal Zn3P2 by physical vapor transport (Figure 1). We are investigating various surface treatment for Zn3P2 to decrease the surface recombination velocity (Figure 2). We are also investigating solar cell performances of Mg/Zn3P2 diodes.
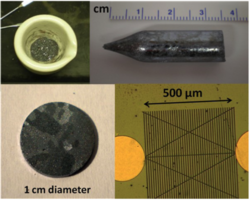
Fig. 1. Preparation of quasi-single crystal Zn3P2 substrates.
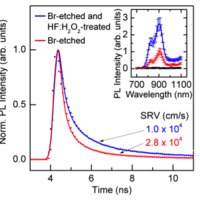
Fig. 2. Photoluminescence (PL) data demonstrate the improved quality of chemically-treated Zn3P2 surfaces. Time-resolved PL decays show a reduction in surface recombination velocity (SRV) and inset shows enhanced steady-state PL from HF:H2O2 treatment.
Cuprous Oxide
Cuprous oxide is a native p-type semiconductor, with a bandgap of 2.0 eV and relatively high absorption coefficient in the visible spectrum range.
We are developing synthesis techniques for preparing high quality Cu2O substrates (Figure 3). We are using semiconductor/liquid contacts to investigate the energy-conversion properties of prepared substrates (Figure 4). We are also interested in exploring other hetero-junction partners for cuprous oxide substrates.
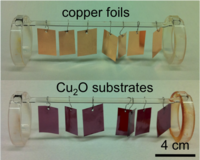
Fig. 3. Synthesis of Cu2O substrates by high temperature thermal oxidation.
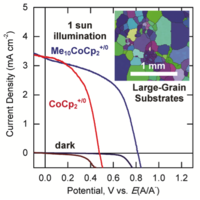
Fig. 4. 820 mV open-circuit voltages were observed from Cu2O photoelectrodes in contact with the decamethylcobaltocene+/0 (Me10CoCp2+/0) redox couple. Inset shows an electron diffraction image of the grain structure in Cu2O substrates.
References
G. M. Kimball, A. M. Muller, N. S. Lewis and H. A. Atwater, “Photoluminescence-based measurements of the energy gap and diffusion length of Zn3P2”,Appl. Phys. Lett. 95, 112103 (2009).
C. Xiang, G. M. Kimball, R. L. Grimm, B. S. Brunschwig, H. A. Atwater, and N. S. Lewis, “820 mV open-circuit voltages from Cu2O/CH3CN junctions”, Energy Environ. Sci.,4, 1311-1318 (2011).
|

