Since early photoelectrochemical studies in the 1970s, TiO2 and WO3 have been discovered to be good photoanode materials for oxidizing water. However, they are not very good light absorbers or charge carrier transporters compared with some of the main-group semiconductors. Our approach to addressing these issues consists of (1) fabrication of nanostructured photoanodes which efficiently collect photogenerated charge carriers; and (2) modification of the band gap of these materials for better absorption of the blue and green portions of the solar spectrum.

Fig. 1. Nanostructured tungsten oxide thin film prepared via anodization.
A widely-used technique to produce metal oxide thin films is anodization. In particular, we have obtained self-organized WO3 films with pore sizes around 100 nm by anodizing tungsten foils in the presence of fluoride ion. The effects of voltage, anodization time, and electrolyte composition on film morphology have been investigated. Preliminary results indicate that porous WO3 electrodes produce significant higher photocurrent densities compared with compact WO3 films, thanks to both streamlined charge collection and enhanced specific surface area.
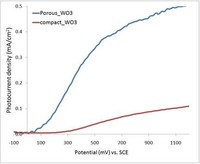
Fig. 2. Comparison of porous and compact tungsten oxide films.
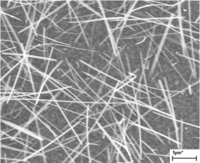
Fig. 3. Tungsten oxide nanowires.
In addition, we have successfully fabricated WO3 nanorod photoanodes via a solvothermal synthesis route and via thermal vapor deposition of WO3 directly onto a substrate material. The WO3 nanorod structure is characterized by techniques such as scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS) and UV-vis spectroscopy. The diameter and length of the prepared WO3 nanorods can be range from 20 to 60 nm and from 1 to 10 µm, respectively. We have been able to incorporate the WO3 nanorod arrays as anodes in photoelectrochemical cells and observe the production of O2 from neutral water under simulated solar illumination.
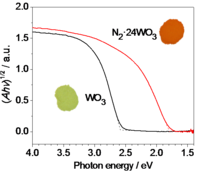
Fig. 4. Band gap modification of tungsten oxide derivatives.
WO3 is an interesting material with a widely variable band gap, from 2.6 eV at room temperate to 1.7 eV at 800 °C. Alternatively, its band gap can be adjusted by fine-tuning its lattice parameters. For example, we have synthesized an orange-red clathrate compound N2 • 24 WO3 isostructural to monoclinic WO3. It features unusual stability and an indirect band gap of 1.8 eV. The guest molecule, 14 N2 or 15 N2, is characterized by compositional analysis, solid-state NMR and Raman spectroscopy, and exhibits a chemical shift and a vibrational frequency close to those of gaseous N2. The origin of the narrow band gap of N2 • 24 WO3 is proposed to be lattice distortions and/or defects due to the inclusion of N2. Currently, we are investigating various techniques to deposit a layer of N2 • 24 WO3 onto a conducting glass electrode, and then we will examine its photoresponse at different excitation wavelengths.

