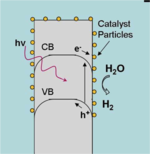
 Most semiconductor surfaces, including that of silicon, are poor catalysts for the hydrogen evolution reaction (HER). Semiconductor water-splitting devices, then, generally require a chemically distinct catalyst species in order to store energy as H-H bonds. Platinum has often been used in the past toward this end, since it catalyzes the HER very efficiently. But due to its low abundance and concomitant high cost, platinum is not desirable for use in a low-cost system.
Most semiconductor surfaces, including that of silicon, are poor catalysts for the hydrogen evolution reaction (HER). Semiconductor water-splitting devices, then, generally require a chemically distinct catalyst species in order to store energy as H-H bonds. Platinum has often been used in the past toward this end, since it catalyzes the HER very efficiently. But due to its low abundance and concomitant high cost, platinum is not desirable for use in a low-cost system.
Another challenge is interfacing the catalyst with a semiconductor surface in a manner that balances light attenuation with sufficient mass loading for overpotential minimization.
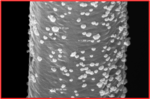 We are examining mixtures of inexpensive transition metals as heterogeneous HER catalysts. This work draws on the extensive literature of pure water electrolysis, from which have emerged a number of promising candidates. We are in the process of developing techniques for depositing known “dark” HER catalysts onto silicon surfaces and understanding the requirements for their efficient function.
We are examining mixtures of inexpensive transition metals as heterogeneous HER catalysts. This work draws on the extensive literature of pure water electrolysis, from which have emerged a number of promising candidates. We are in the process of developing techniques for depositing known “dark” HER catalysts onto silicon surfaces and understanding the requirements for their efficient function.

