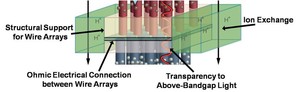
Fig. 1. Schematic of the membrane layer of the solar fuel generation device.
The membrane layer between the two semiconductor electrodes must fulfill a number of important functions for the device to split water sustainably (Fig. 1). It must simultaneously provide structural support for the wire arrays, separate the gaseous hydrogen and oxygen products, enable an ohmic conduction path for electrons between the anode and cathode, and act as an ion-exchange medium for the protons involved in the electrochemical reaction, all while being optically transparent enough to ensure that light is effectively absorbed by both semiconductor assemblies. The Lewis group is exploring materials and fabrication routes to meet this daunting challenge. Arrays of p-Si wire arrays have already been successfully transferred to low-cost polydimethylsiloxane (PDMS) layers, resulting in thin, flexible, transparent, structurally robust films.1 These polymer-supported Si wire arrays have been employed in liquid-junction photoelectrochemical cells as well, yielding reasonably high energy-conversion efficiencies.2

Fig. 2. Si wire arrays embedded in thin Nafion films. The array (a) is uniform over a large area, (b) can be peeled from the substrate in a thin Nafion film, © can be tranferred with most of the wire length exposed from the Nafion, and (d) makes intimate contact with the polymer. Scale bar is 100 µm for (a,b), 20 µm for ©, and 2 µm for (d).
In its essential functions, the envisioned water-splitting membrane acts as a proton exchange membrane (PEM) fuel cell operating in reverse. The membrane of PEM fuel cells must also be impermeable to hydrogen and oxygen gases while allowing efficient proton exchange. The material most commonly used in these membranes is perfluorosulphonic acid polytetrafluoroethylene copolymer, known as Nafion. Nafion is commercially available and can be highly transparent when cast from solution. Current research efforts are focused on transferring wire arrays into proton-conductive, gas-impermeable, transparent, structurally-robust Nafion films with controllable exposure of the semiconductor wire length from the membrane. Such films have been produced (Fig. 2), and their properties are being characterized.
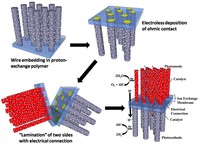
Fig. 3. Schematic of the membrane fabrication process.
Nafion, however, does not fulfill all the required characteristics of the membrane by itself. In particular, it is not electrically conductive. In future Lewis group efforts to make the full device (Fig. 3), each wire array would be peeled in a Nafion film, thin layers of an appropriate metal would be electrolessly deposited on the back of the wire bases to establish an ohmic contact, and the two sides of the membrane would be carefully sealed together using a third, electrically conducting Nafion layer. Work is under way between the Lewis group and the Freund group at the University of Manitoba to make the middle layer of Nafion conducting by the addition of conjugated polymer chains (i.e., PEDOT) to the extent permitted without substantial degradation of the transparency or ion-exchange capability of this thin film.

