Silicon Surface Chemistry
Many semiconductor photovoltaics do not have stable surfaces with respect to oxidation in air or liquids. Silicon, in particular, spontaneously forms oxides at its surface that degrade photovoltaic and photoelectrochemical performance. Thus, the goals of the surface chemistry subgroup are:
Modify surfaces to reduce the degree of surface oxidation with chemically stable species.
Use surface functionalization to modify photoelectrochemical device energetics.
Leverage our new surface functionality to covalently attach catalysts for reactions such as hydrogen production.
To accomplish these goals, the Lewis group employs synthetic organic techniques to build hydrocarbon surfaces that address these issues. However, there are still several issues to be addressed and several avenues of research available to young researchers.
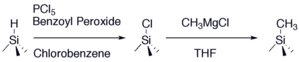
Fig. 1. The two-step chloronation-alkylation procedure forms a stable methyl-terminated surface that demonstrates long-term air stability and high silicon photoelectrochemical performance.
Methyl-Terminated Surfaces
The Lewis group pioneered research in functionalizing silicon surface with small organic species that accomplish the goals stated above. For instance, we demonstrated a two-step synthesis illustrated in Figure 1 that methylates silicon surfaces with close to complete coverage that effectively inhibits oxidation. Electrochemical methods and instrumental techniques such as XPS and SRV effectively charcterize the quality, performance, and stability of these surfaces.1-3
While the methyl-terminated surface is highly resistant to chemical and photooxidation reactions, it is unfortunately also resistant to subsequent functionalization chemistry we would like to employ to further improve performance. To that end, we have pioneered research into using mixed surface functionality to balance these two goals.
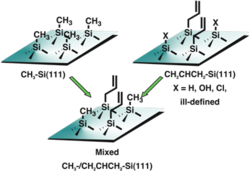
Fig. 2. Methyl surfaces provide chemical stability but no route to further functionalization. Functionalized surfaces with larger moieties such as alkenes do not completely cover the surface and lead to unwanted oxidation and degradation of photoelectrochemical performance. The mixed monolayer surface has the potential to accomplish both goals of resistance to oxidation but the capability of further productive chemistry.
Mixed Monolayer Surfaces
Methyl termination of silicon surfaces is an effective barrier towards oxidation, unfortunately it is also an effective barrier towards further useful chemistry. In many cases, simply adding larger species with more chemical functionalities (such as an alkene) does not result in complete coverage of the silicon surface. In this case the surface oxidizes and the performance degrades accordingly. A mixed monolayer surface represents a potential solution to this problem, as depicted in Figure 2.
Recently our two-step synthesis was effectively extended by introducing two Grignard species in the second step in Figure 1. In Figure 3, the allyl Grignard enables secondary surface modification, while the methyl Grignard fills in any unreacted sites to form a surface with complete organic coverage, and partial allyl functionality. Further, by spreading the allyls throughout the surface, they are not as sterically congested which in turn facilitates secondary chemistry.
Current work in the Lewis group is focused on applying this technique to make surfaces functionalized with sterically constrained groups and catalyst linking agents with enhanced surface properties while lowering the time and cost involved in production.
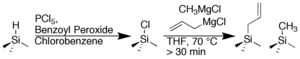
Fig. 3. Synthesis of a mixed monolayer surface.
Functionalizing Structured Silicon Surfaces
Much of the work described above has been performed on well-defined silicon surfaces such as Si(111). However, recent work in our group, the Atwater group, and others suggests that structured silicon surfaces may be a route to more cost-effective and practical photovoltaic and photoelectrochemical devices than the well-ordered Si(111) surface. To that end, we are currently investigating how this chemistry behaves on these silicon structures.
Analytical Techniques
Students in the Lewis group are exposed to a wide range of research and academic disciplines to accomplish there goals. Further, students in the Surfsci subgroup additionally have the opportunity to play with instruments such as the XPS, SRV, IR, AFM, and others to investigate the properties of the surfaces we generate.
XPS – X-ray Photoelectron Spectroscopy
SRV – Surface Recombination Velocity
IR – Infrared Spectroscopy
AFM – Atomic Force Microscopy
References
Hamann, T. W.; Lewis, N. S. J Phys Chem B 2006, 110, 22291.
Jaeckel, B.; Hunger, R.; Webb, L. J.; Jaegermann, W.; Lewis, N. S. J Phys Chem C 2007, 111, 18204.
Maldonado, S.; Plass, K. E.; Knapp, D.; Lewis, N. S. J Phys Chem C 2007, 111, 17690.
|

